Rescue after high-dose methotrexate therapy in patients with osteosarcoma1
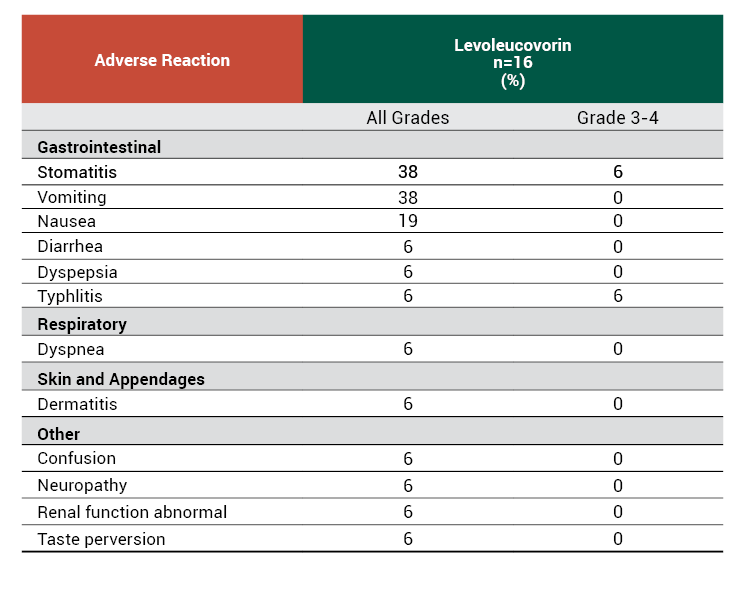
The efficacy of levoleucovorin rescue following high-dose methotrexate were evaluated in 16 patients, aged 6 to 21 years, who received 58 courses of therapy for osteogenic sarcoma. High-dose methotrexate was one component of several different combination chemotherapy regimens evaluated across several trials. Methotrexate 12 g/m2 as an intravenous infusion over 4 hours was administered to 13 patients, who received levoleucovorin 7.5 mg by intravenous infusion every 6 hours for 60 hours or longer beginning 24 hours after completion of methotrexate. Three patients received methotrexate 12.5 g/m2 intravenously over 6 hours, followed by levoleucovorin 7.5 mg by intravenous infusion every 3 hours for 18 doses beginning 12 hours after completion of methotrexate. The mean number of levoleucovorin doses per course was 18.2 and the mean total dose per course was 350 mg. The efficacy of levoleucovorin rescue following high-dose methotrexate was based on adverse reaction profile.
Adverse Reactions With High-Dose Methotrexate Therapy
Dosing and Administration
KHAPZORY® is indicated for intravenous administration only. Do not administer intrathecally.
The recommended dosage for KHAPZORY® is based on a methotrexate dose of 12 grams/m2 administered as intravenous infusion over 4 hours. Twenty-four hours after starting the methotrexate infusion, initiate KHAPZORY® at a dose of 7.5 mg (approximately 5 mg/m2) as an intravenous infusion every 6 hours.
Monitor serum creatinine and methotrexate levels at least once daily. Continue KHAPZORY®, hydration, and urinary alkalinization (pH of 7 or greater) until the methotrexate level is below 5 x 10-8 M (0.05 micromolar). Adjust the dose or extend the duration as recommended in Table 1.
Table 1 Recommended Dosage for KHAPZORY® Based on Serum Methotrexate and Creatinine Levels

Impaired Methotrexate Elimination or Renal Impairment
Decreased methotrexate elimination or renal impairment which are clinically important but less severe than the abnormalities described in Table 1 can occur following methotrexate administration. If toxicity associated with methotrexate is observed, in subsequent courses extend KHAPZORY® rescue for an additional 24 hours (total of 14 doses over 84 hours).
Third-Space Fluid Collection and Other Causes of Delayed Methotrexate Elimination
Accumulation in a third space fluid collection (i.e., ascites, pleural effusion), renal insufficiency, or inadequate hydration can delay methotrexate elimination. Under such circumstances, higher doses of KHAPZORY® or prolonged administration may be indicated.
KHAPZORY® Important Safety Information
Indications and Usage
KHAPZORY® is a folate analog indicated for the treatment of adults with metastatic colorectal cancer in combination with fluorouracil, as rescue after high-dose methotrexate therapy in adult and pediatric patients with osteosarcoma, and for diminishing the toxicity associated with overdosage of folic acid antagonists or impaired methotrexate elimination in adult and pediatric patients.
Limitations of Use
KHAPZORY® (levoleucovorin) is not indicated for the treatment of pernicious anemia and megaloblastic anemia secondary to lack of vitamin B12 because of the risk of progression of neurologic manifestations despite hematologic remission.
Important Safety Information
Contraindications
- KHAPZORY® is contraindicated in patients who have had severe hypersensitivity to leucovorin products, folic acid, or folinic acid.
Warnings and Precautions
- Increased Gastrointestinal Toxicities with Fluorouracil: Gastrointestinal toxicities, including stomatitis and diarrhea, occur more commonly and may be of greater severity and of prolonged duration. Deaths from severe enterocolitis, diarrhea, and dehydration have occurred in elderly patients receiving weekly d,l-leucovorin and fluorouracil. Do not initiate or continue therapy with KHAPZORY® and fluorouracil in patients with symptoms of gastrointestinal toxicity until those symptoms have resolved. Monitor patients with diarrhea until it has resolved as rapid deterioration leading to death can occur.
- Drug Interaction with Trimethoprim-Sulfamethoxazole: Concomitant use of d,l-leucovorin with trimethoprim-sulfamethoxazole for the acute treatment of Pneumocystis jiroveci pneumonia in patients with HIV infection increased treatment failure and morbidity.
Adverse Reactions
- The most common adverse reactions (≥20%) in patients receiving high-dose methotrexate therapy with levoleucovorin rescue were stomatitis (38%) and vomiting (38%).
- The most common adverse reactions (>50%) in patients receiving levoleucovorin in combination with fluorouracil for metastatic colorectal cancer were stomatitis (72%), diarrhea (70%), and nausea (62%).
Drug Interactions
Leucovorin products increase the toxicity of fluorouracil.
Use in Specific Population
There is limited data with levoleucovorin use in pregnant women. KHAPZORY® is administered in combination with methotrexate or fluorouracil, which can cause embryo-fetal harm. Refer to methotrexate and fluorouracil Prescribing Information for additional information.
Reporting of Suspected Adverse Reactions
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Reference:
- KHAPZORY®. Prescribing Information. Acrotech Biopharma Inc.