Fluorouracil inhibits DNA synthesis1,2
Treatment with fluorouracil alone
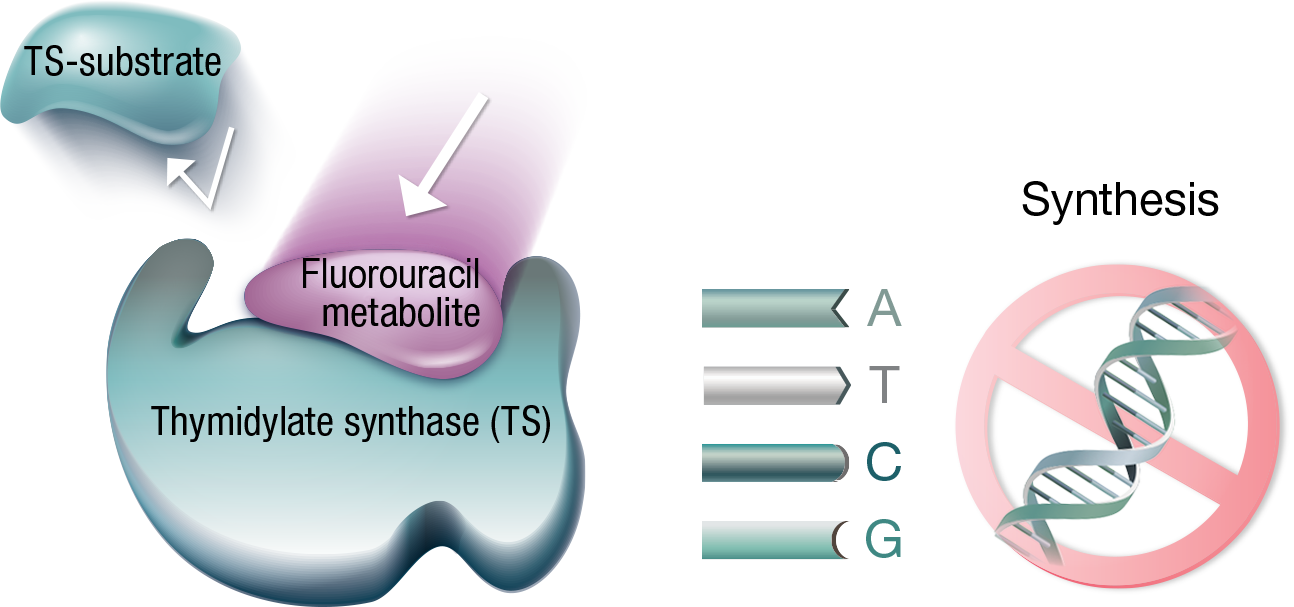
Thymidylate synthase (TS) is a key enzyme in the creation of thymine, a building block of DNA. Fluorouracil competes with the natural substrate to bind the TS active site. When bound to TS, fluorouracil inhibits its activity, arresting DNA synthesis.
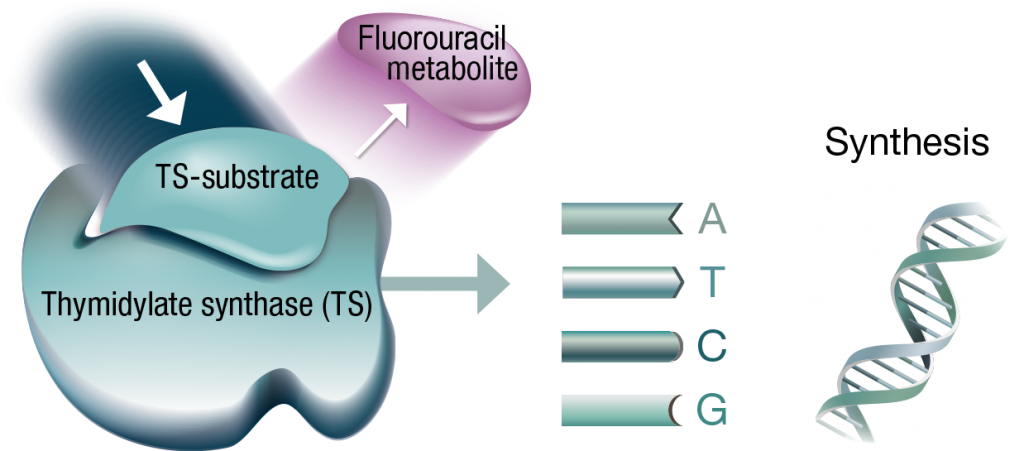
However, fluorouracil alone can be displaced, enabling DNA synthesis in cancer cells to continue.
5-MTHF stabilizes fluorouracil metabolite to block DNA synthesis1,3
Treatment with fluorouracil + KHAPZORY®
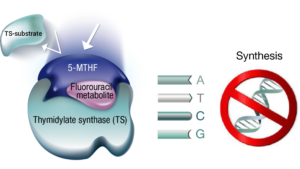
Levoleucovorin is readily converted to 5,10-methylenetetrahydrofolate (5-MTHF), which stabilizes the bond between the fluorouracil metabolite and thymidylate synthase, enhancing the inhibition of this enzyme.
Note: Mechanism of action pathway has been simplified for illustrative purposes.
KHAPZORY® Important Safety Information
Indications and Usage
KHAPZORY® is a folate analog indicated for the treatment of adults with metastatic colorectal cancer in combination with fluorouracil, as rescue after high-dose methotrexate therapy in adult and pediatric patients with osteosarcoma, and for diminishing the toxicity associated with overdosage of folic acid antagonists or impaired methotrexate elimination in adult and pediatric patients.
Limitations of Use
KHAPZORY® (levoleucovorin) is not indicated for the treatment of pernicious anemia and megaloblastic anemia secondary to lack of vitamin B12 because of the risk of progression of neurologic manifestations despite hematologic remission.
Important Safety Information
Contraindications
- KHAPZORY® is contraindicated in patients who have had severe hypersensitivity to leucovorin products, folic acid, or folinic acid.
Warnings and Precautions
- Increased Gastrointestinal Toxicities with Fluorouracil: Gastrointestinal toxicities, including stomatitis and diarrhea, occur more commonly and may be of greater severity and of prolonged duration. Deaths from severe enterocolitis, diarrhea, and dehydration have occurred in elderly patients receiving weekly d,l-leucovorin and fluorouracil. Do not initiate or continue therapy with KHAPZORY® and fluorouracil in patients with symptoms of gastrointestinal toxicity until those symptoms have resolved. Monitor patients with diarrhea until it has resolved as rapid deterioration leading to death can occur.
- Drug Interaction with Trimethoprim-Sulfamethoxazole: Concomitant use of d,l-leucovorin with trimethoprim-sulfamethoxazole for the acute treatment of Pneumocystis jiroveci pneumonia in patients with HIV infection increased treatment failure and morbidity.
Adverse Reactions
- The most common adverse reactions (≥20%) in patients receiving high-dose methotrexate therapy with levoleucovorin rescue were stomatitis (38%) and vomiting (38%).
- The most common adverse reactions (>50%) in patients receiving levoleucovorin in combination with fluorouracil for metastatic colorectal cancer were stomatitis (72%), diarrhea (70%), and nausea (62%).
Drug Interactions
Leucovorin products increase the toxicity of fluorouracil.
Use in Specific Population
There is limited data with levoleucovorin use in pregnant women. KHAPZORY® is administered in combination with methotrexate or fluorouracil, which can cause embryo-fetal harm. Refer to methotrexate and fluorouracil Prescribing Information for additional information.
Reporting of Suspected Adverse Reactions
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
References:
- Khapzory®. Prescribing Information. Acrotech Biopharma Inc.
- Zittoun J. Annals of Oncol. 1993;4(Suppl 2):S1-S5.
- Peters GJ, et al. Biochem Biophys Acta. 2002;1587(2-3):194-205.

